How do we decide which studies to host? ....
New Study Feasibility and Prioritisation Process
We host research studies across many of our clinical specialities within our Hospitals. For more information please see Research Areas. The R&D Department received on average over 10 new study opportunities for circulation and consideration each week. These come from a variety of sources including those sent through from the NIHR Clinical Research Network, directly from study Sponsors or from clinicians. For around 10% of these new study opportunities there is an Expression of Interest made by a member of staff to host the study in the Trust. Therefore, the R&D Department must ensure due consideration takes place for all new Expressions of Interest. To facilitate this all* Expressions of Interest will go through the same systematic feasibility and prioritisation process as described in Figure 1.
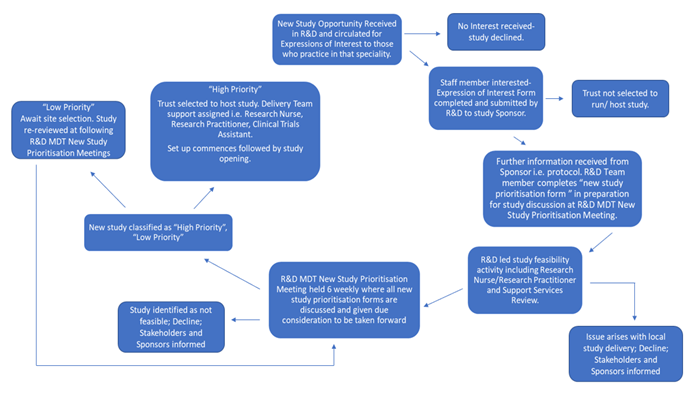
Figure 1. New Study Feasibility and Prioritisation Process.
For each new study this process considers the comments of the potential Principle Investigator, Delivery Teams, i.e. Research Nurses/Research Practitioners/Clinical Trials Assistants, Laboratory and Pharmacy personnel, Senior Management and Research Delivery Facilitators. There is also a requirement to consider the High-Level Objectives set by the NIHR Clinical Research Network in relation to things such as the potential for Recruitment to Time and Target given available resources.
At the end of the feasibility and prioritisation process each new study will be classified as either High Priority, Low Priority or Declined. Only when a new study has been classified as High Priority will local study set up commence, which will be initiated by the Research Delivery Facilitators. Given the high volume of new studies, there are times when some may have to be Declined by the R&D Department and this may be due to one or more factors relating to those considerations noted above.
For more information please contact the Research Delivery Facilitators via yhs-tr.Research.Governance@nhs.net
*This does not include those projects that are being undertaken as part of an educational qualification or that are designed and Sponsored by the Trust. These studies would automatically proceed through to set up.