Meet The Team
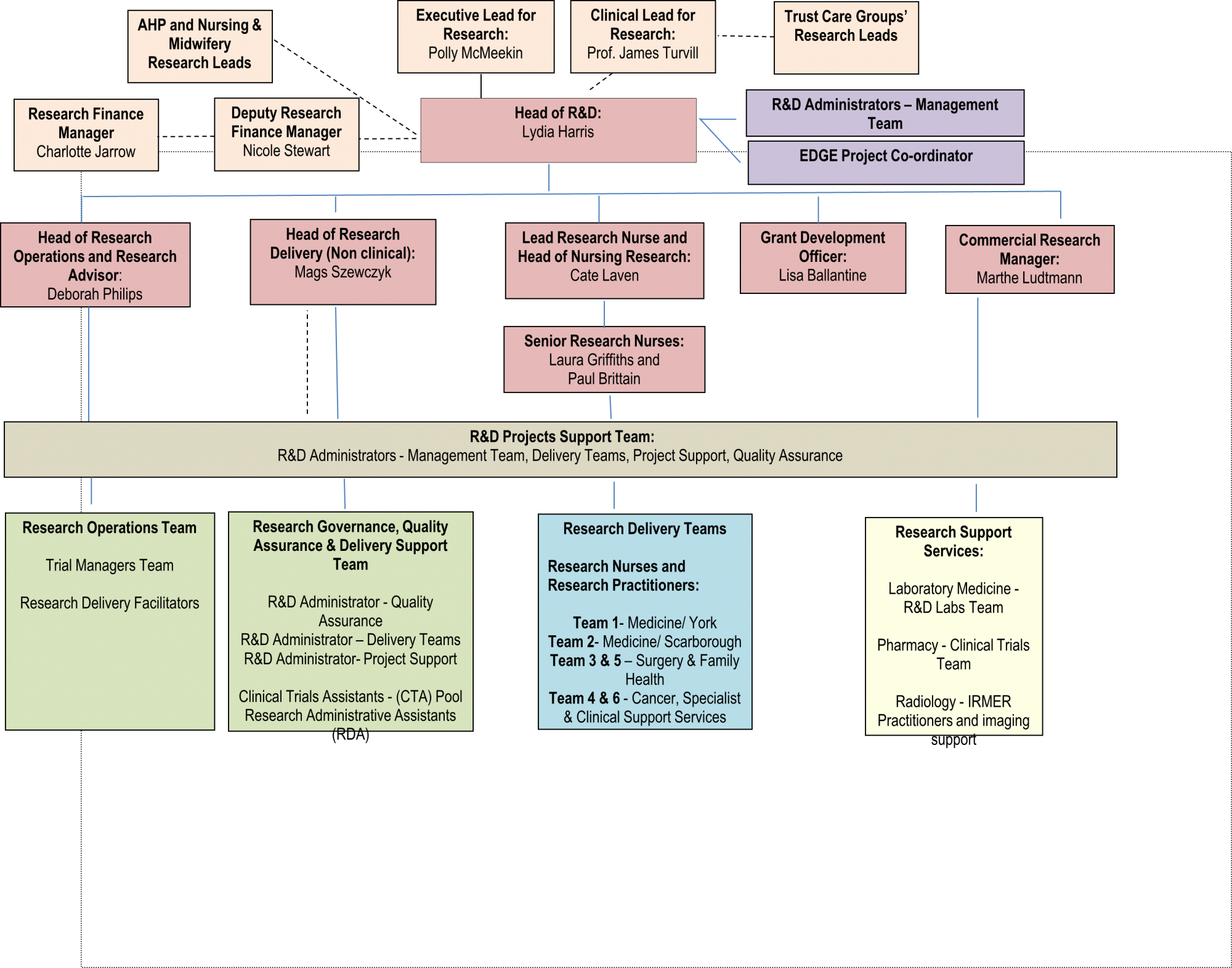
The R&I Department, headed up by Lydia Harris (Head of R&I), supports a wide range of research functions. We have a large clinical delivery workforce made up of Research Nurses (RNs), Research Practitioners (RPs) and Clinical Trials Assistants (CTAs). In addition we have a Research Management and Support Team overseeing the governance, contractual and financial arrangements for research, the management of studies, and quality assurance and oversight function. A dedicated Commercial Manager oversees all aspects of commercial research and a Research Grant Development Officer is able to support funding applications. Our experienced Trial Management team are available to manage all aspects of study approval and delivery. All are supported by an Administrative team alongside dedicated Laboratory and Pharmacy research teams. Each of our Hospital's Care Groups has a Clinical Research Lead and our Clinical Director for Research and Innovation is Professor James Turvill.
Please click on the names of the Teams below the Organogram to find out more about us and how to contact us: